BY: SAMANTHA BARTLETT, DVM
Applied DNA Sciences and EvviVax have been approved by the U.S. Department of Agriculture and New York State Department of Agriculture and Markets to conduct a trial for a vaccine against COVID-19 in cats.
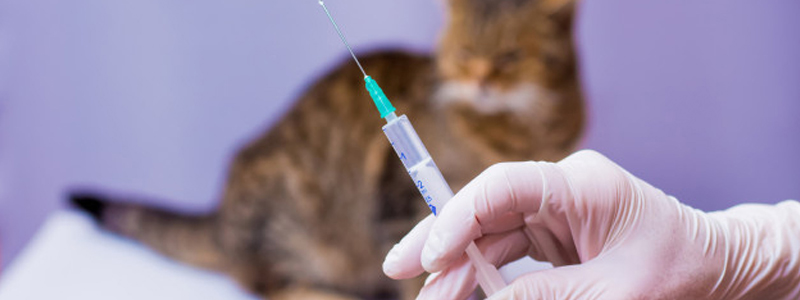
The trial will follow 15 healthy client-owned cats for six months during and after vaccination. The cats will receive two doses of vaccine given 30 days apart. The companies will monitor the safety of the vaccine and the cats’ immune responses over the 6 month period.
Applied DNA Sciences and EvviVax believe cats could be a potential reservoir for human infections. Another concern is that domestic cats could eventually transmit the virus to wildlife forming an uncontrolled reservoir of infection. There are no cases known where cats have transmitted the virus to people. In the approximately 40 known cases of COVID-19 in cats, people were suspected as the source of infection. Regardless, cats currently do not seem to be affected in significant numbers and most have mild symptoms.
If the trial is successful, the companies will perform another trial challenging the vaccine against the virus. Then, they will apply for conditional approval from USDA’s Animal and Plant Health Inspection Service so they can offer the vaccine on the commercial market. The vaccine might be attractive to zoos with populations of large cats Cases of COVID-19 in big cats, including tigers, lions, and a puma, have been documented at the Bronx and Knoxville Zoos and a zoo in Johannesburg.
The President and CEO of Applied DNA Sciences, James A. Hayward, expects that the trials for the feline vaccine will also help by providing useful data for human COVID-19 vaccine trials. It may also help toward the development of a vaccine for other animals, particularly mink. Mink farms have been particularly hard hit by the COVID-19 pandemic and many farms have had to cull their populations.
The USDA currently does not feel that COVID-19 vaccines for pets are an urgent need and are not granting any approvals for COVID-19 vaccines. They are, however, accepting applications for vaccines for minks. Zoetis is in talks with USDA on the development of a mink vaccine. Zoetis believes this vaccine can be rapidly adapted for dogs and cats in the future.